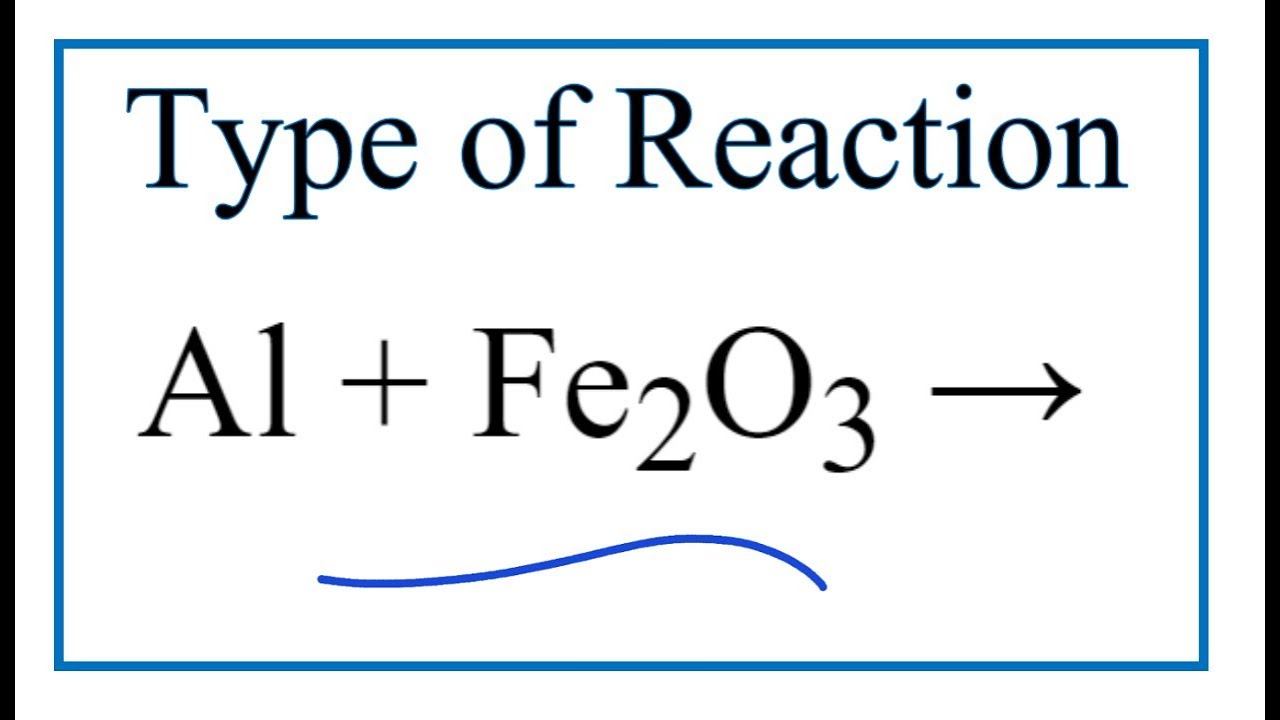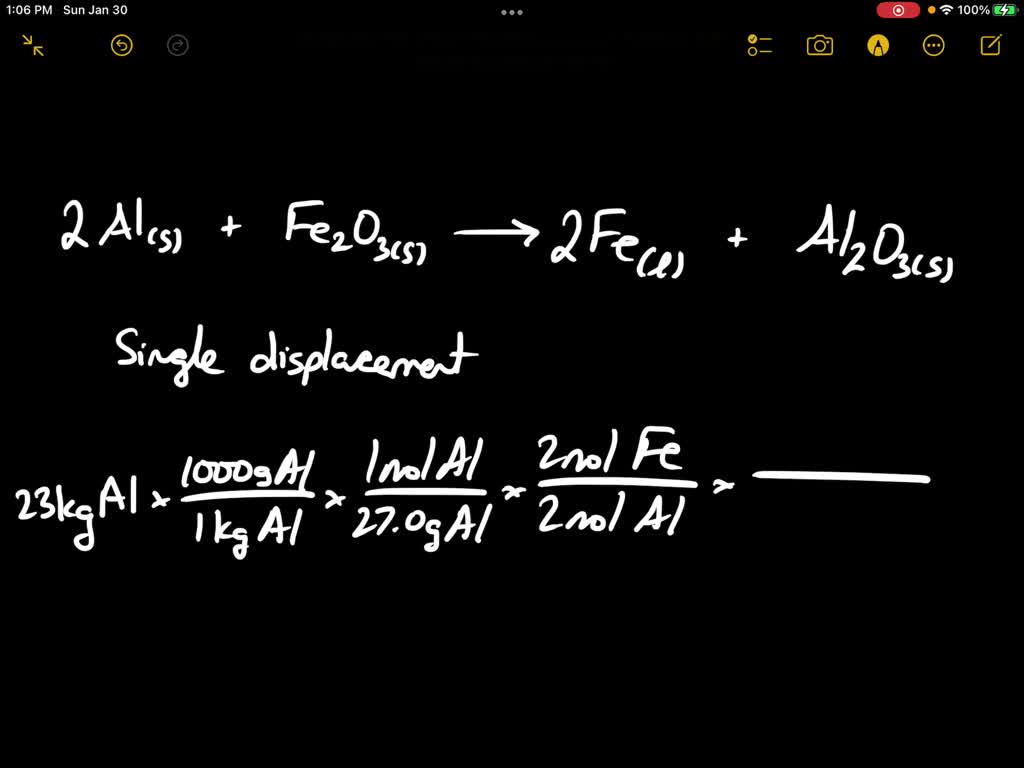
SOLVED: Solid aluminum reacts with solid iron (III) oxide to produce liquid iron and aluminum oxide powder. Write a balanced chemical equation for this reaction showing the reactants and products. Include subscripts
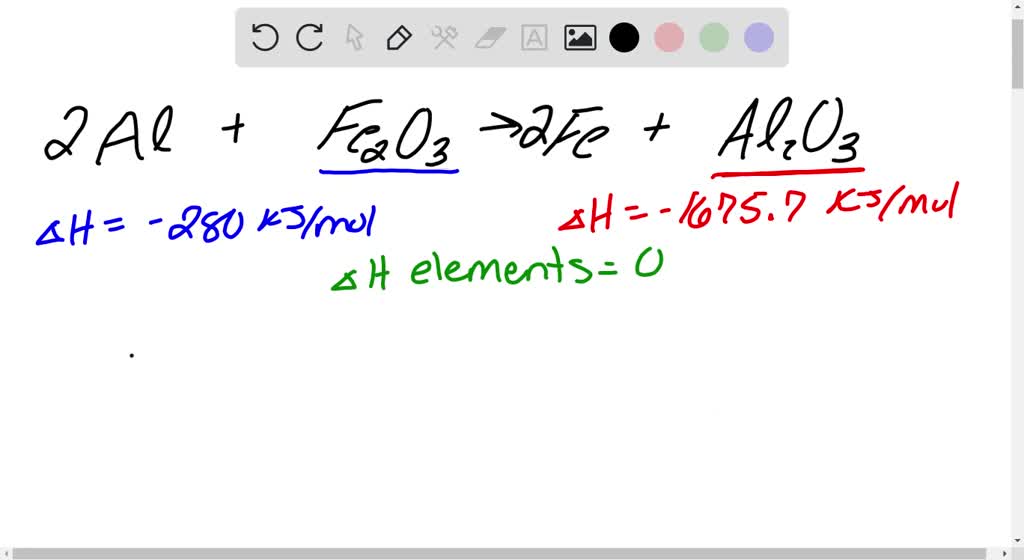
SOLVED: When aluminium metal reacts with iron (III) oxide a single displacement reaction occurs to form iron and aluminum oxide. Using the Standard Enthalpy Chart write the balanced chemical equation and calculate

XRD pattern of iron aluminum oxide nanoparticles for oxidation time of... | Download Scientific Diagram

Types of corrosion that affect rolling stock and how to reduce exposure | Sponsored | Railway Gazette International
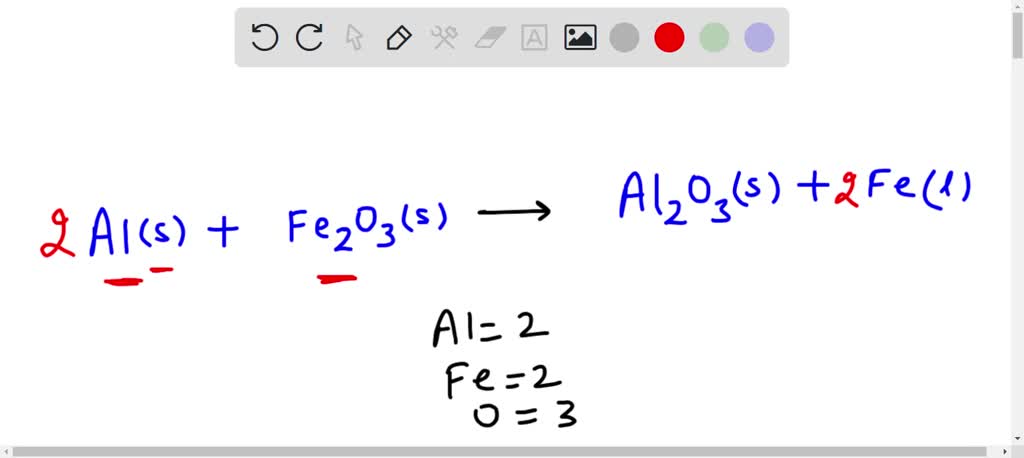
SOLVED: Write a balanced chemical equation based on the following description: the reaction of powdered aluminum and powdered iron(III) oxide produces solid aluminum oxide and liquid iron metal

Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils — A myth revisited - ScienceDirect