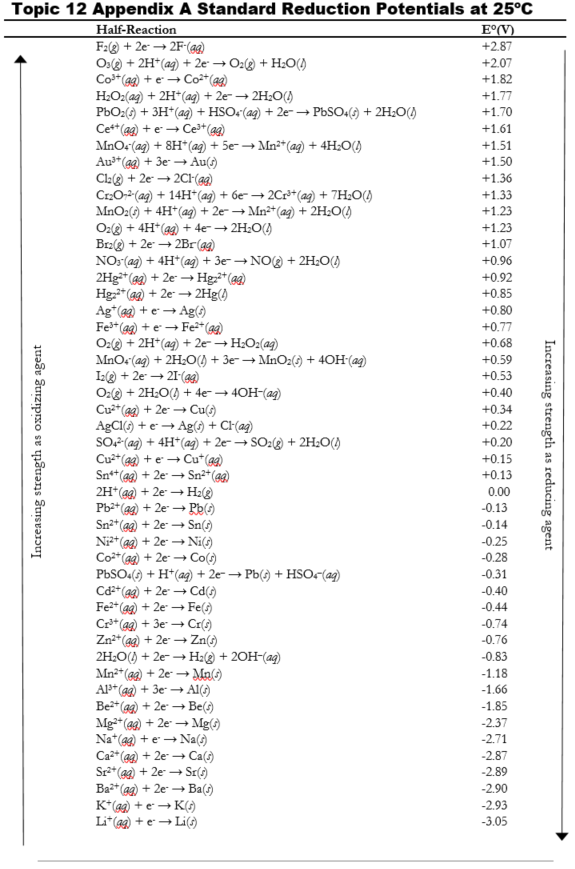
zYF0s-nkAniXrg0gJSMR9_mugFa0Gv1ajkfDRlJ8WQ0CcOw2u4K7JnIXP1VAsmnWjpKnjrAtJ6MVhADt9pZGKM2fwU1VbeaZ9iKLGR4OWswN35PeJH77qNaEiJjfwjwyWw
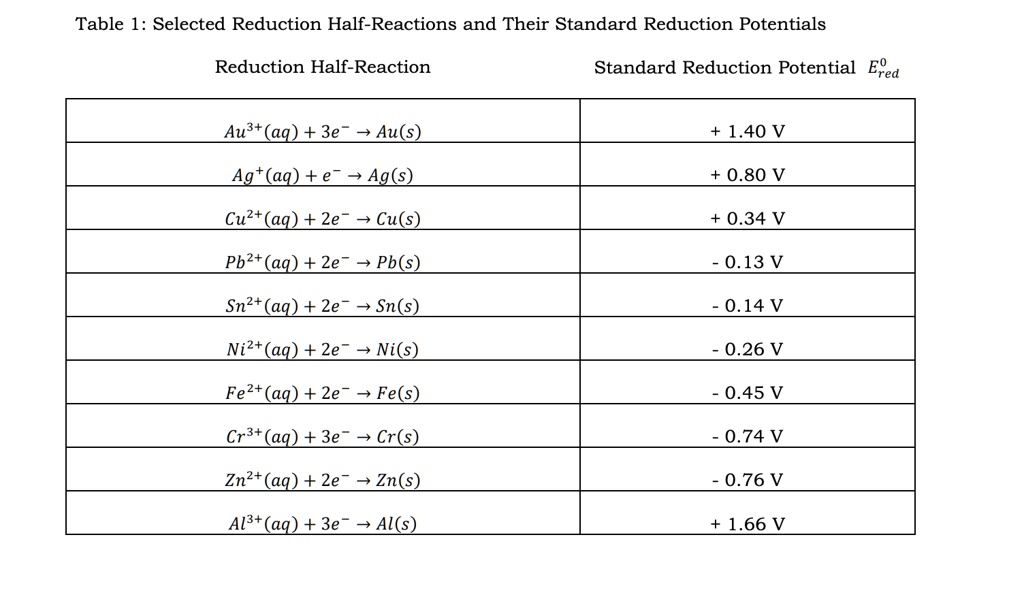
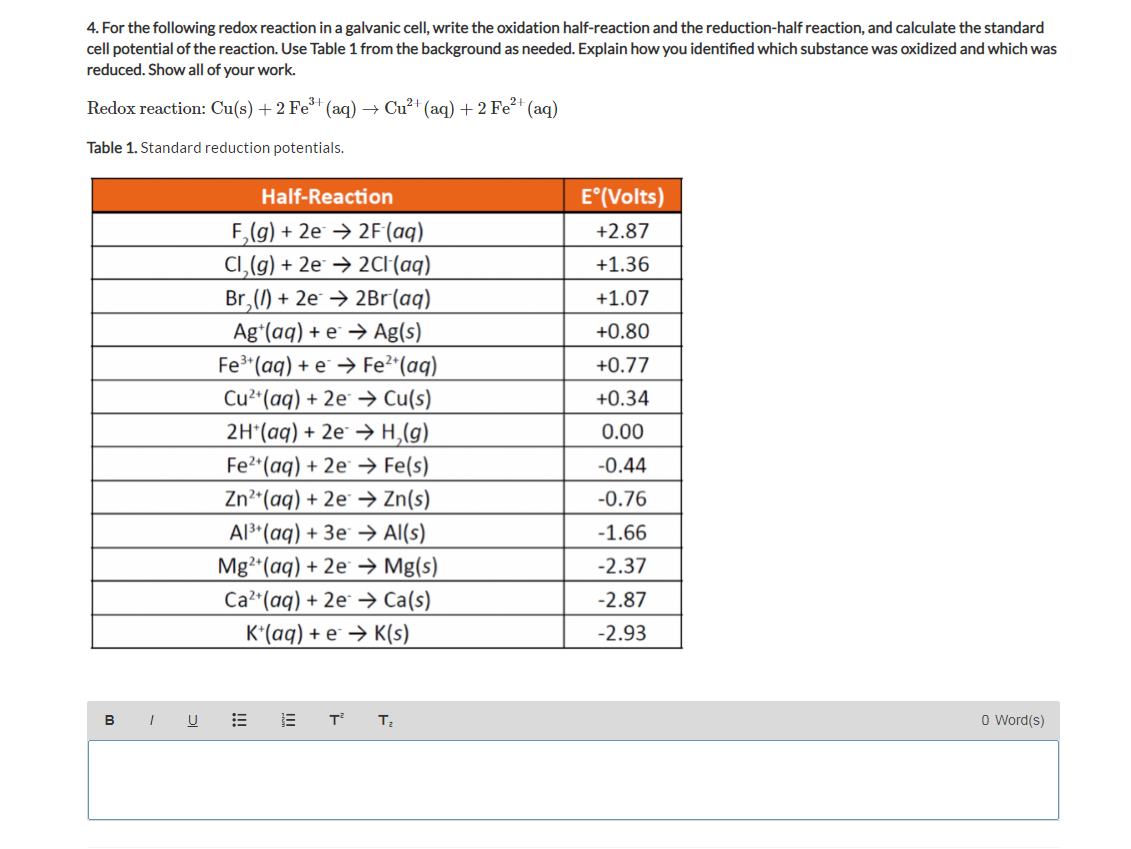
SOLVED: Table 1: Selected Reduction Half-Reactions and Their Standard Reduction Potentials Reduction Half-Reaction Standard Reduction Potential Ered Au3+ (aq) + 3e Au(s) 1.40 V Agt(ag) +e Ag(s) 0.80 V Cuz+ (ag) +

physical chemistry - Explain why can't zinc be plated out from a Zn(II) solution using standard reduction potentials - Chemistry Stack Exchange

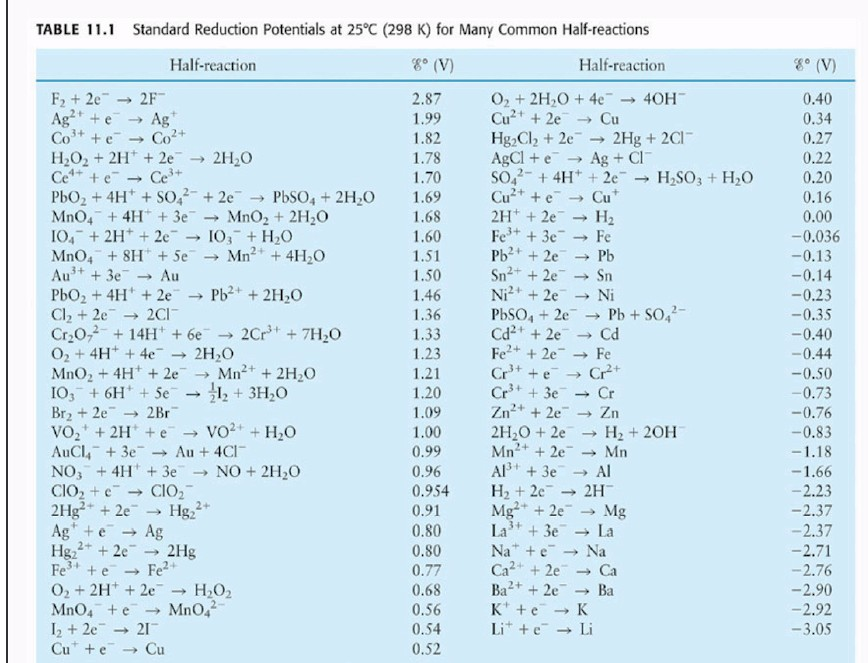
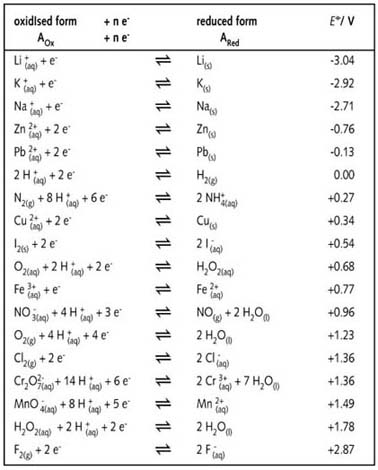
SOLVED: Table A5.5 Standard Reduction Potentials at 258C (298 K) for Many Common Half-Reactions Half-Reaction Half-Reaction 4OH Fz + 2e 2F 2.87 01 2H,0 + 4e Cu? + Ze Cu Ag + +

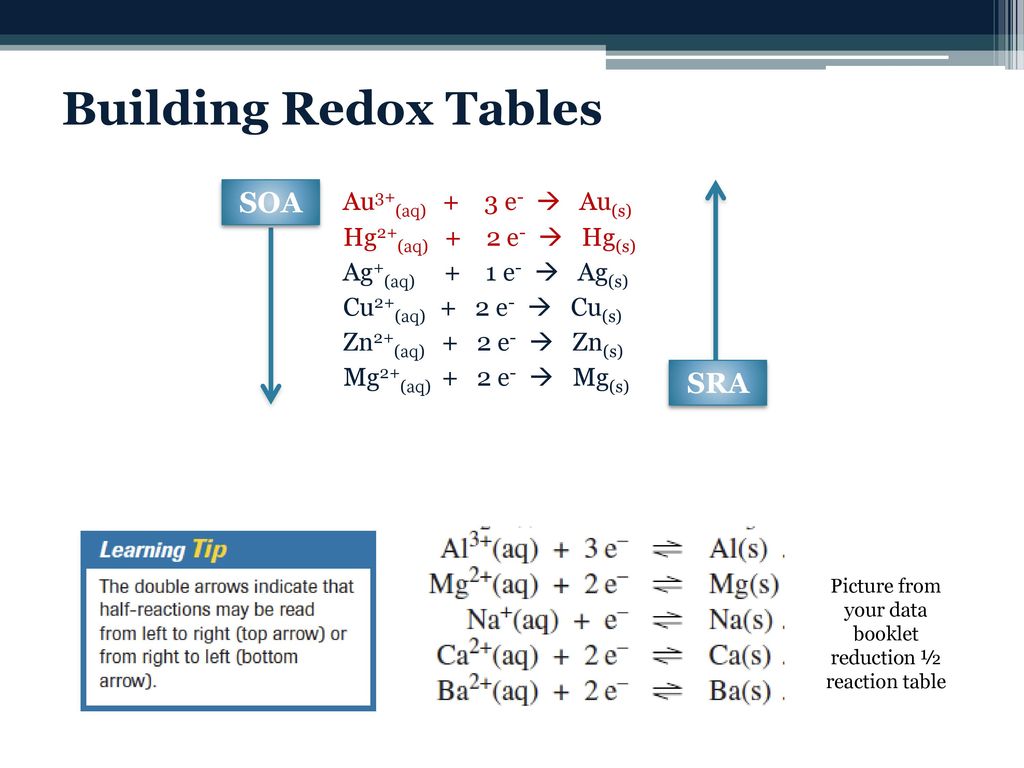
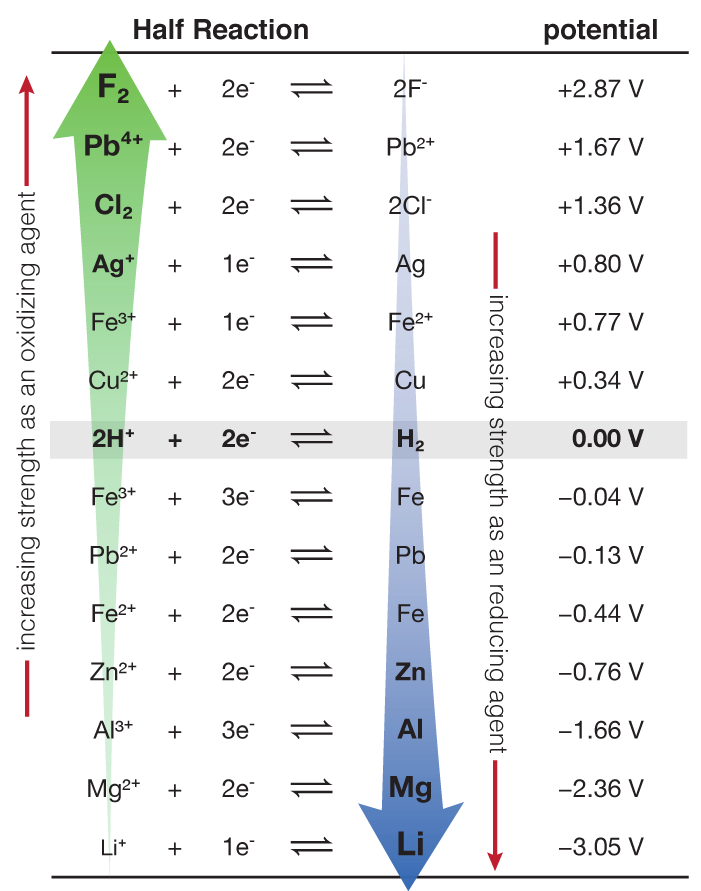
Gr. 12 Science: Redox reactions R E D O X Red uction Gaining of electrons (e - ) The oxidizing agent undergoes reduction Ox idation Loss of electrons (e. - ppt download
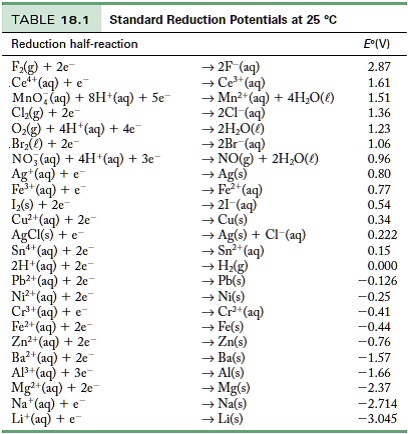
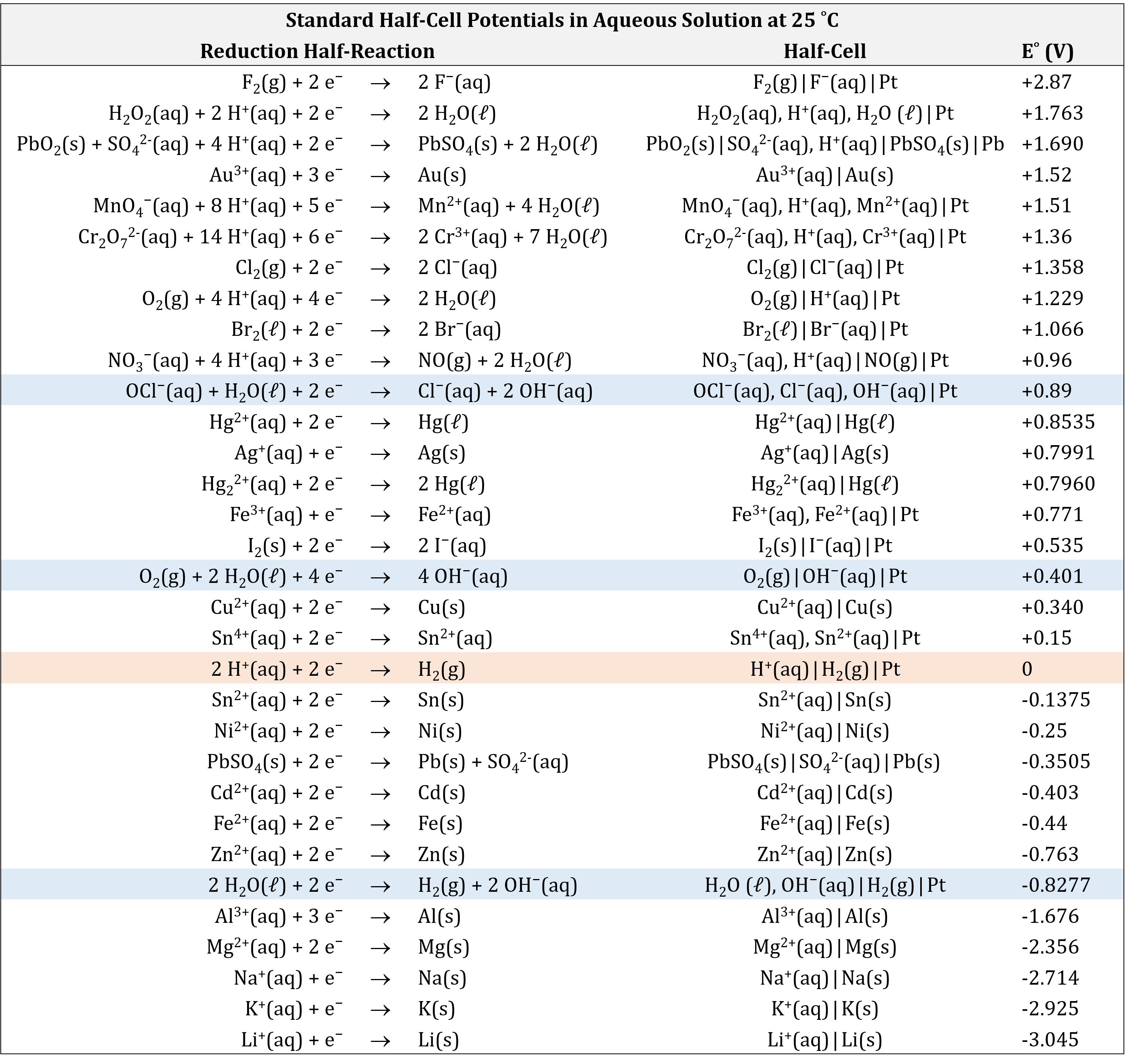

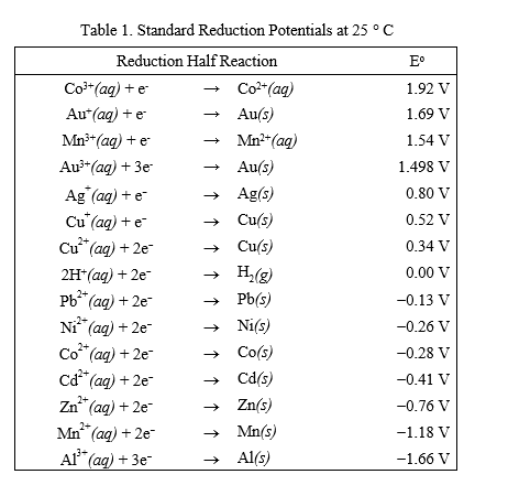

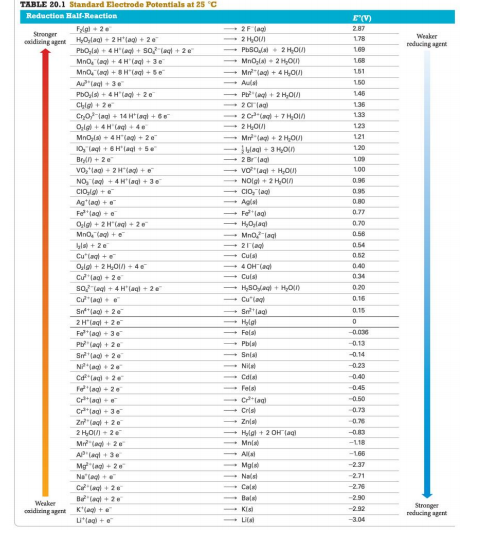
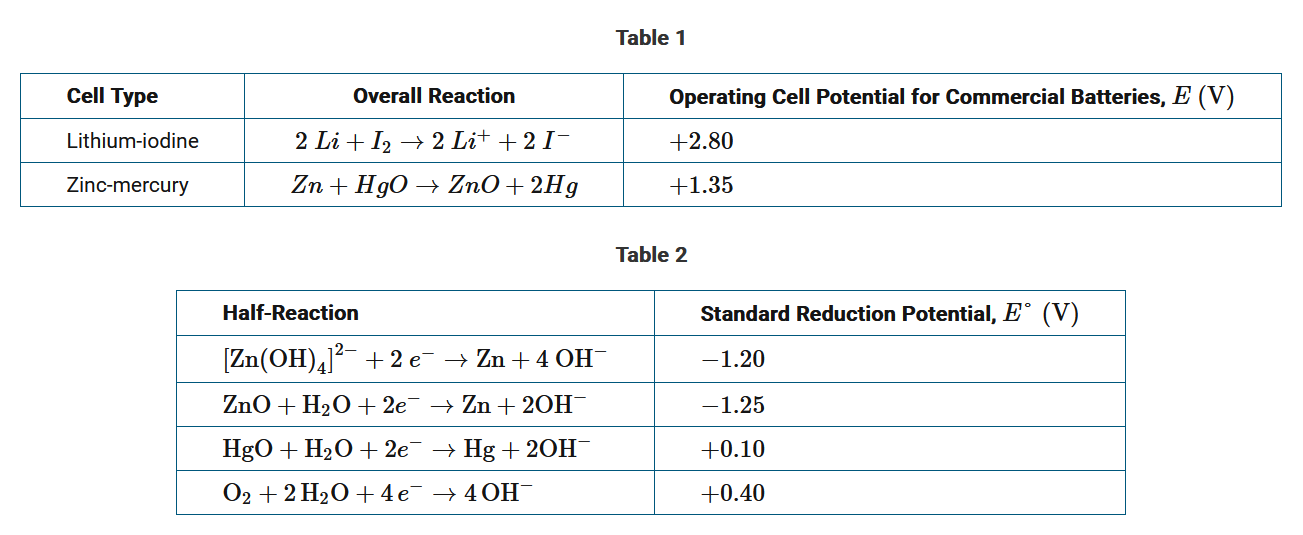
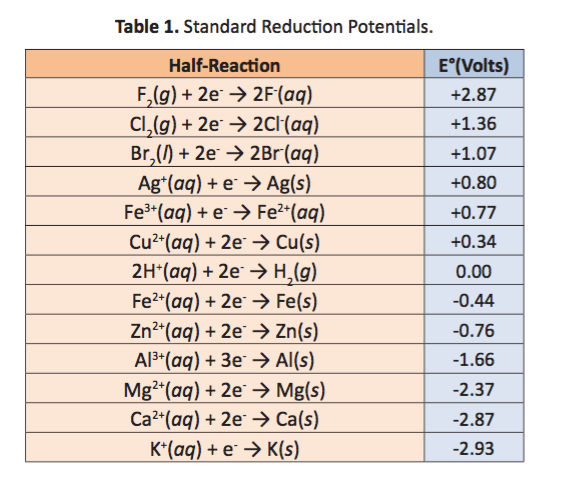
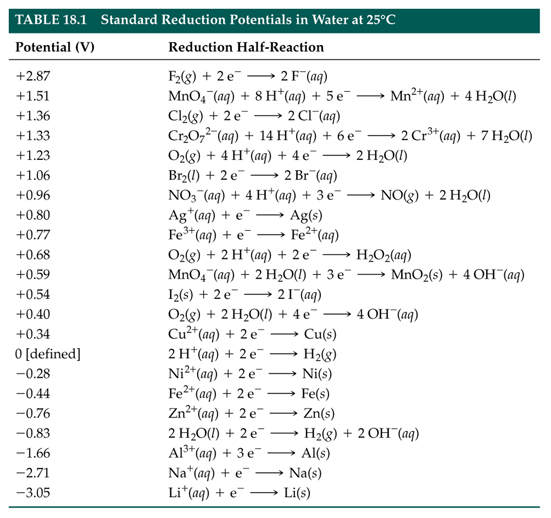

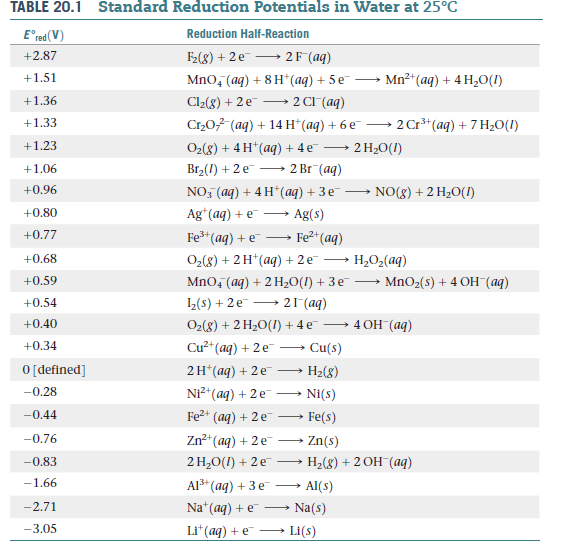
![Solved] Using data from Table, place the followin | SolutionInn Solved] Using data from Table, place the followin | SolutionInn](https://s3.amazonaws.com/si.question.images/image/images7/442-E-C-E-E-C(12).png)