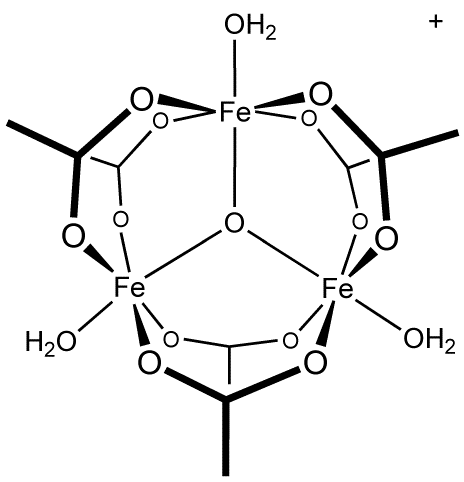
A “Star” Antiferromagnet: A Polymeric Iron(III) Acetate That Exhibits Both Spin Frustration and Long‐Range Magnetic Ordering - Zheng - 2007 - Angewandte Chemie International Edition - Wiley Online Library

Table 1 from Formation peculiarities of iron (III) acetate: potential precursor for iron metal-organic frameworks (MOFs) | Semantic Scholar

Figure 2 from Formation peculiarities of iron (III) acetate: potential precursor for iron metal-organic frameworks (MOFs) | Semantic Scholar
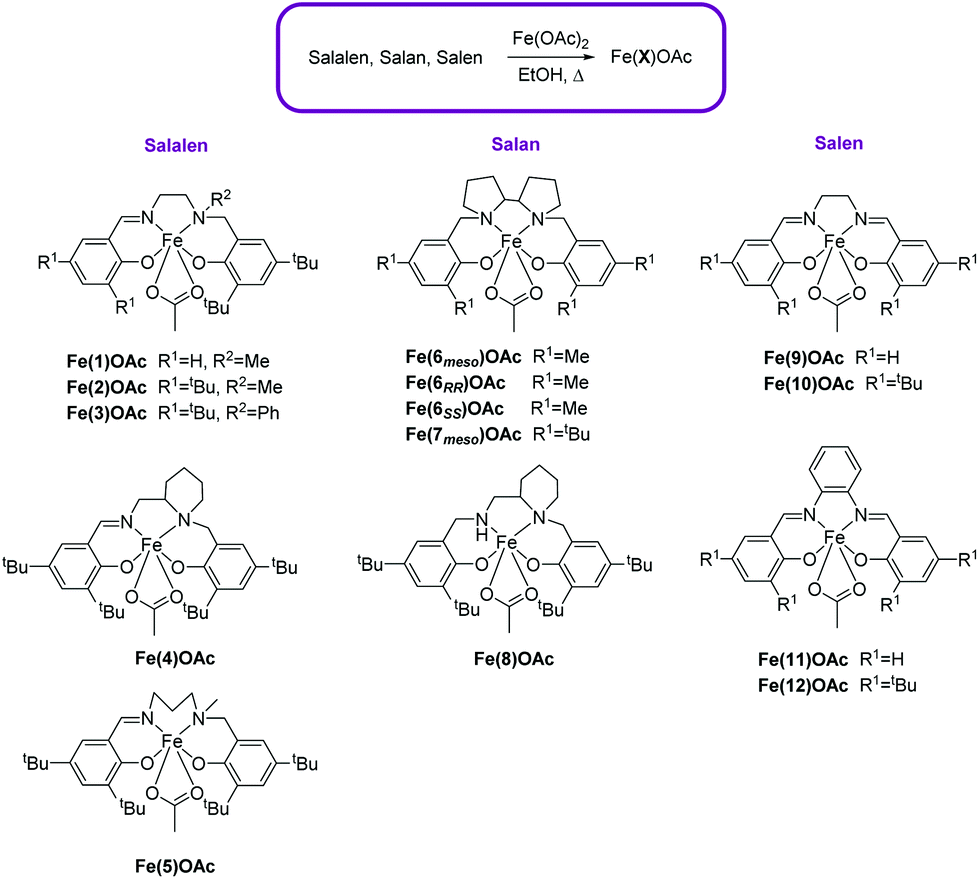
The synthesis, characterisation and application of iron(iii)–acetate complexes for cyclic carbonate formation and the polymerisation of lactide - Dalton Transactions (RSC Publishing)
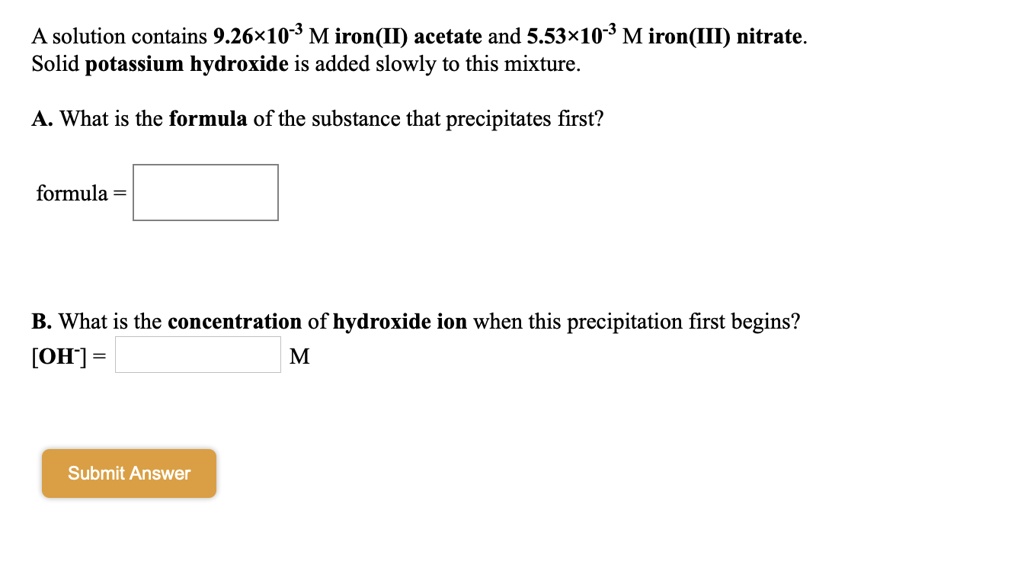
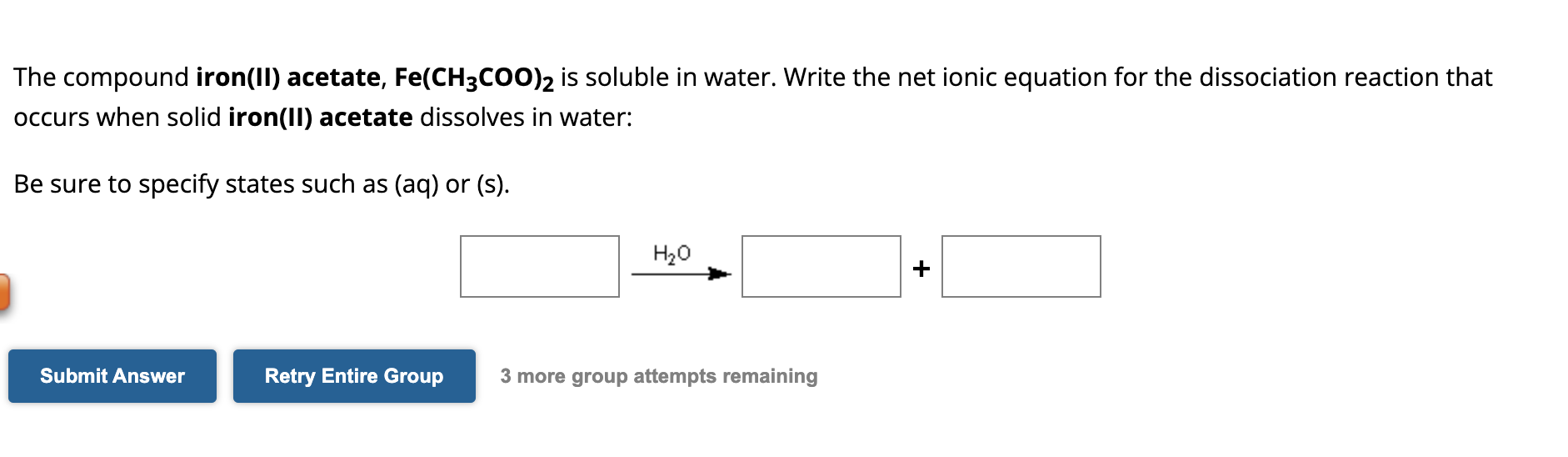
SOLVED: A solution contains 9.26*10-* M iron(II) acetate and 5.53x10-* M iron(III) nitrate. Solid potassium hydroxide is added slowly to this mixture What is the formula of the substance that precipitates first?

Figure 1 from Formation peculiarities of iron (III) acetate: potential precursor for iron metal-organic frameworks (MOFs) | Semantic Scholar

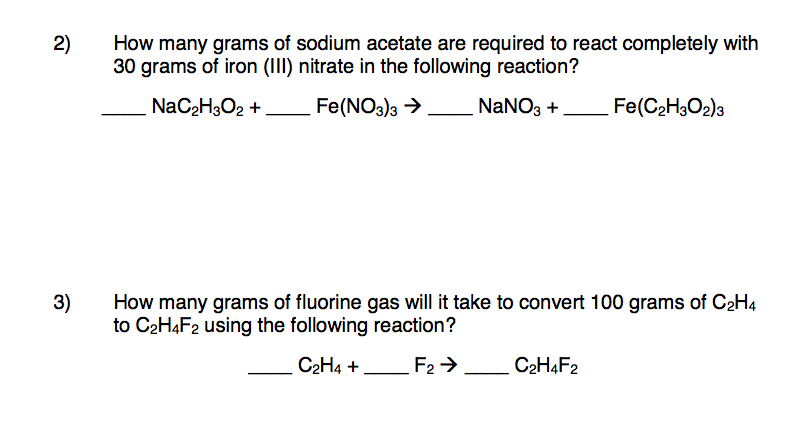
SOLVED: sodium sulfide Iron (III) acetate => ukcul ALale oL 6EEEuLd Total 3NA ,2/44 ) 4 Fc (c#aCoo) , (64) Cl+z @ONBla ) + FtzS3(5) Ionic Aa "(a4) + 35?-(a4)+ 2Fe < (
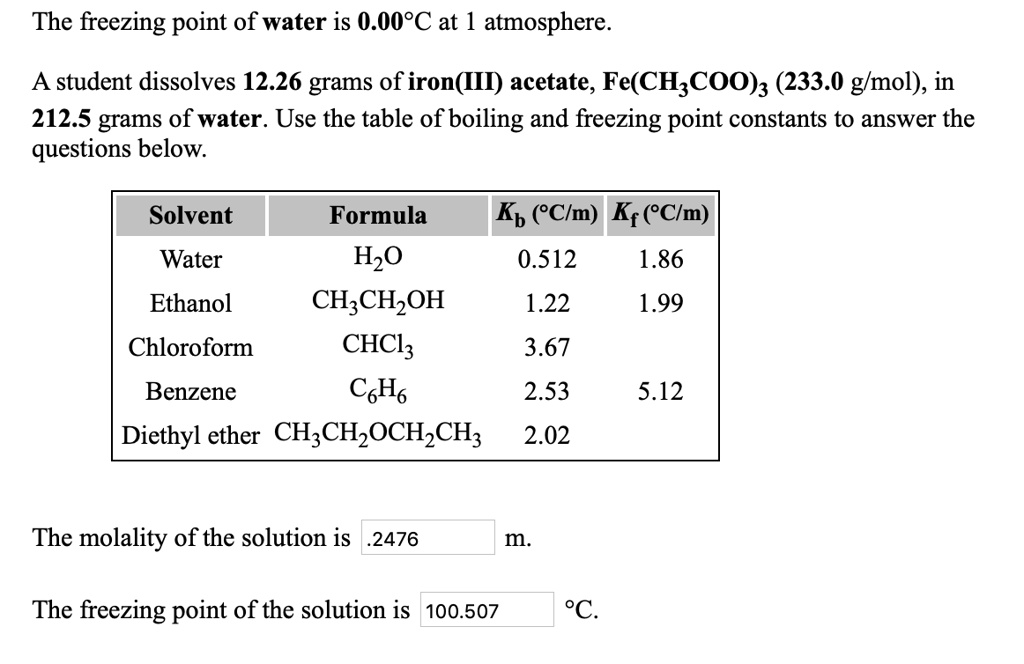
SOLVED: The freezing point of water is 0.00PC at 1 atmosphere A student dissolves 12.26 grams of iron(III) acetate, Fe(CH;COO)z (233.0 glmol), in 212.5 grams of water. Use the table of boiling