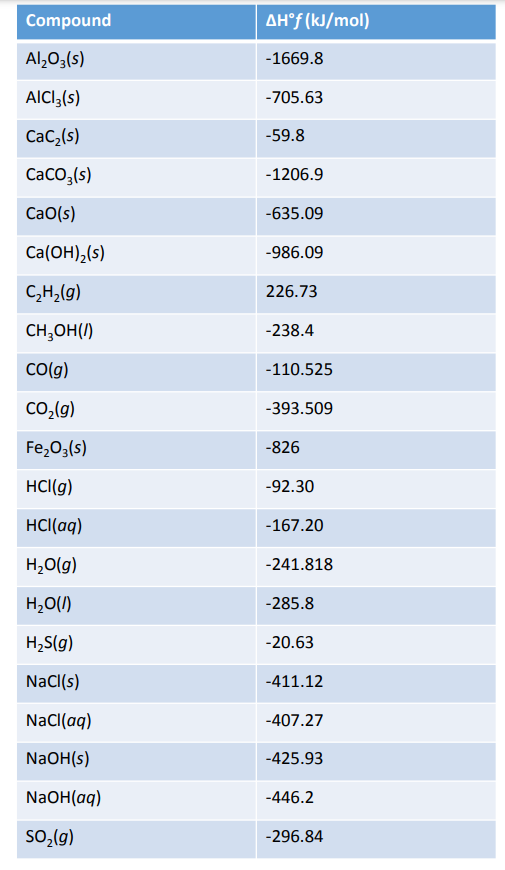
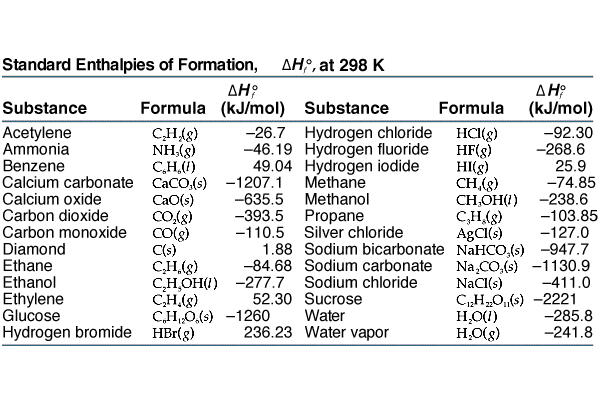
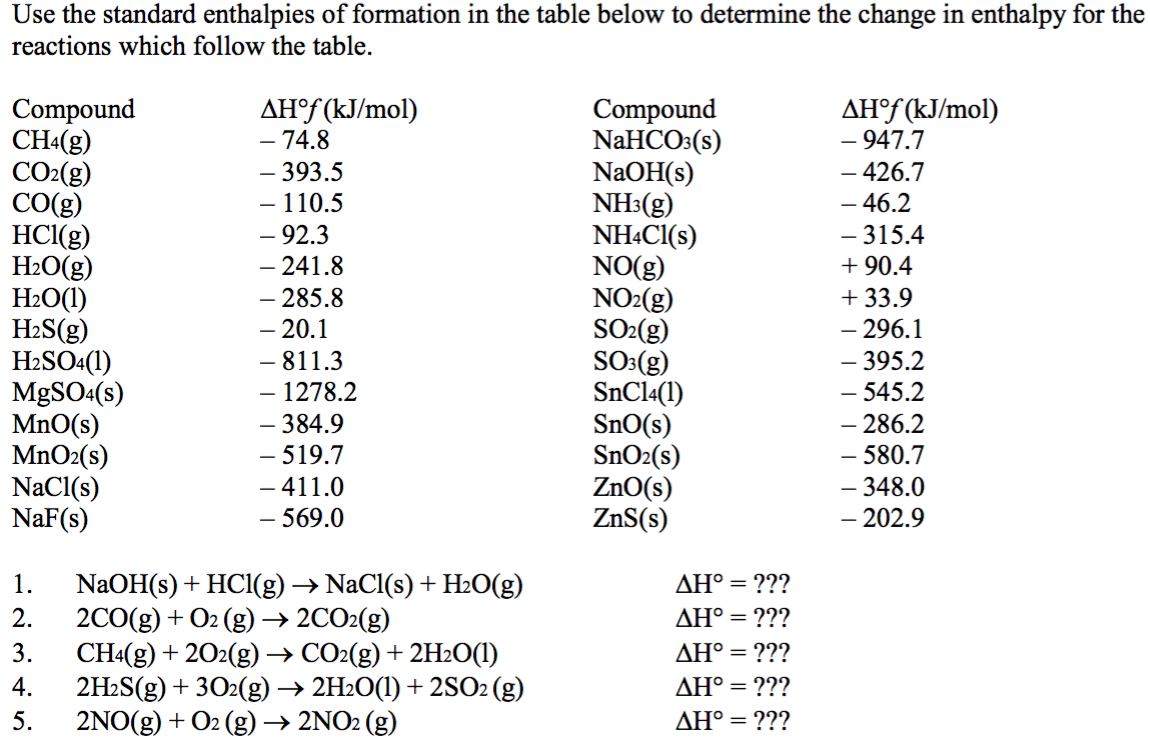
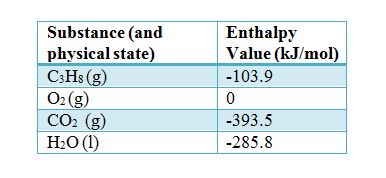
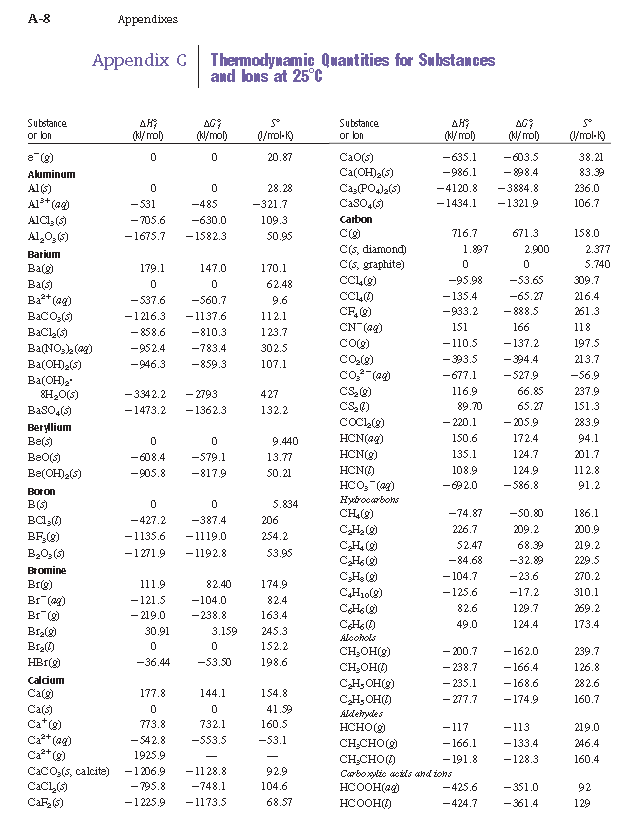
![Appendix D. Heats of Formation and Combustion - Basic Principles and Calculations in Chemical Engineering [Book] Appendix D. Heats of Formation and Combustion - Basic Principles and Calculations in Chemical Engineering [Book]](https://www.oreilly.com/api/v2/epubs/9780132885478/files/graphics/appd-tab-d1a.jpg)
Appendix D. Heats of Formation and Combustion - Basic Principles and Calculations in Chemical Engineering [Book]

Using the table for standard enthalpy of formation, solve 2 CO (g) + O2 (g) --> 2 CO2 (g) - Brainly.com
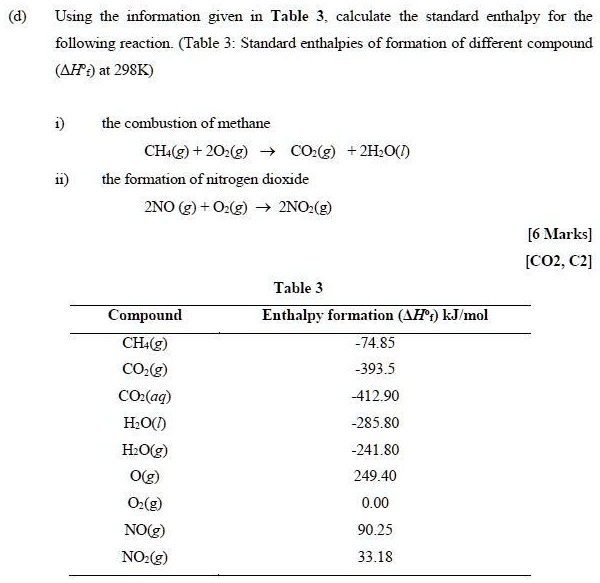
SOLVED: Using the information given Table 3 calculate the standard enthalpy for the following reaction (Table 3: Standard enthalpies of formation of different compound (AF:) at 298K) the combustion of methane CH(g) +

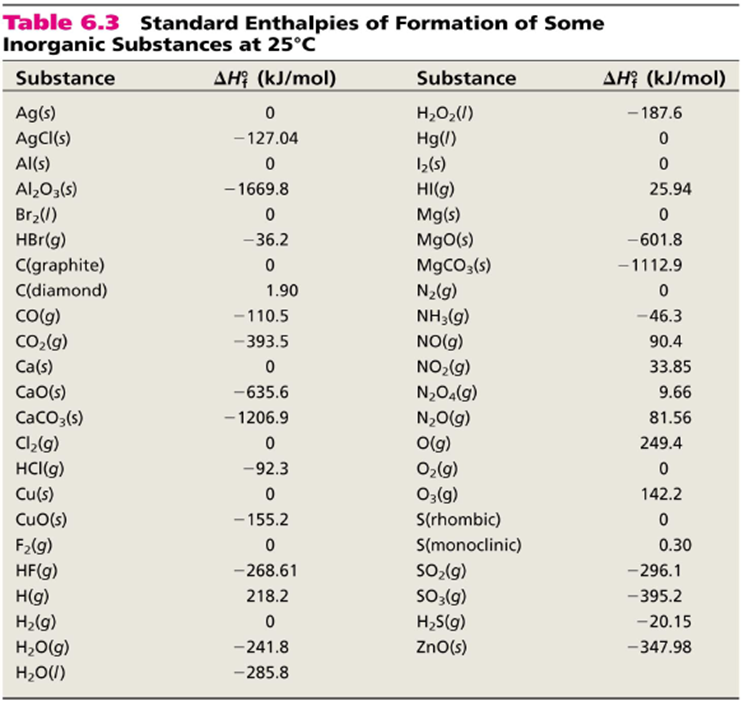
Standard Enthalpies of Formation Table .pdf - Table 3.4 Standard Enthalpies and Gibbs Energies of Formation at 298.15 K 25CJT Joules per mole of the | Course Hero

Table 3 from Group additivity values for enthalpies of formation (298 K), entropies (298 K), and molar heat capacities (300 K < T < 1500 K) of gaseous fluorocarbons | Semantic Scholar

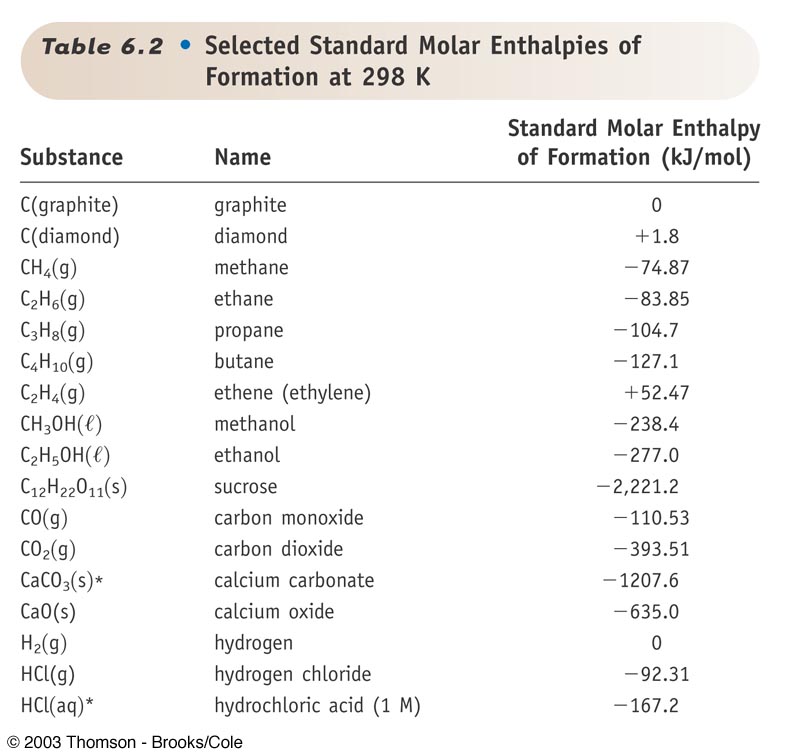
2009, Prentice-Hall, Inc. Enthalpies of Formation An enthalpy of formation, H f, is defined as the enthalpy change for the reaction in which a compound. - ppt download
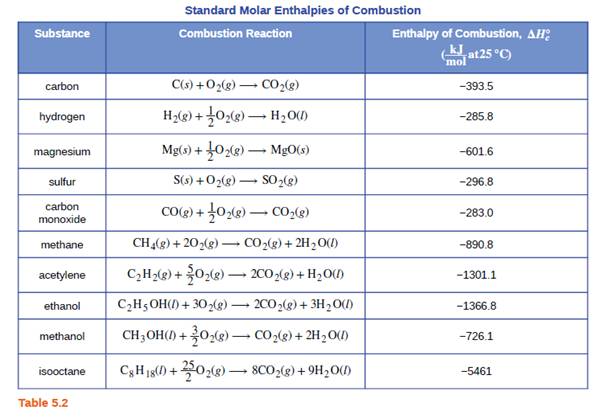
Which of the enthalpies of combustion in Table 5.2 the table are also standard enthalpies of formation? | bartleby

Correlation between Standard Enthalpy of Formation and Refractive Index in Alkali Halides | Semantic Scholar

Table VII from Large-scale calculations of gas phase thermochemistry: Enthalpy of formation, standard entropy, and heat capacity | Semantic Scholar